WHIM syndrome: a combined primary immunodeficiency with heterogeneous presentation1,2
In the largest cohort study of people with WHIM syndrome, only ~23% of patients presented with all 4 features in the WHIM acronym (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis).2
Prevalence of WHIM Syndrome-Related Manifestations in an International Cohort (N=66)*,2
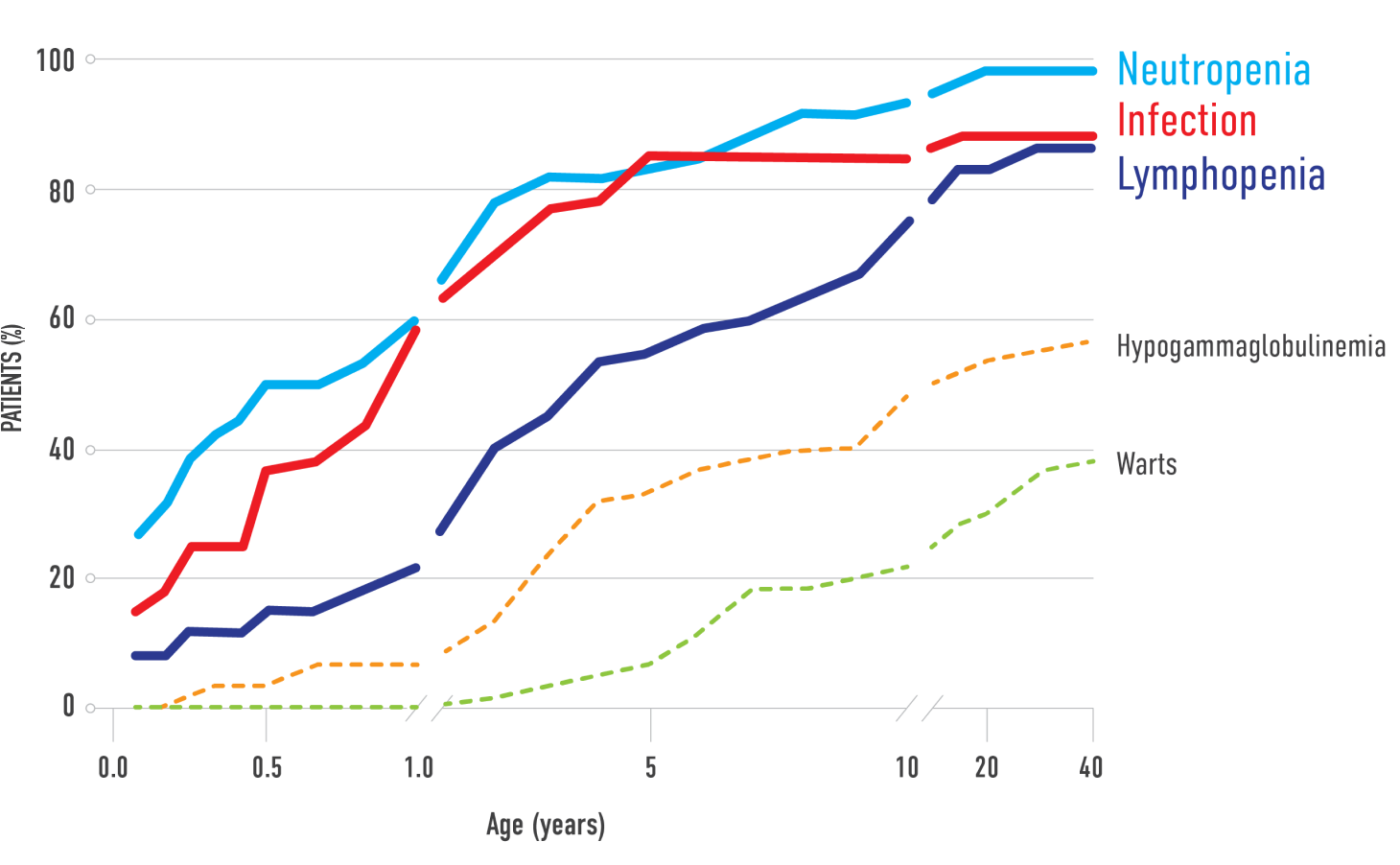
Immunodeficiency in WHIM syndrome leads to an increased susceptibility to recurrent and severe bacterial and viral infections.2,3
*Cumulative percentage of WHIM syndrome manifestations at a given age.
†Lifetime prevalence of individual WHIM syndrome-related manifestations and laboratory findings (frequency as % total cases).
Most common manifestations†,2:
- 98% - Neutropenia
- 92% - Infection
- 88% - Lymphopenia
Immunodeficiency in WHIM syndrome leads to an increased susceptibility to recurrent and severe bacterial and viral infections.2,3
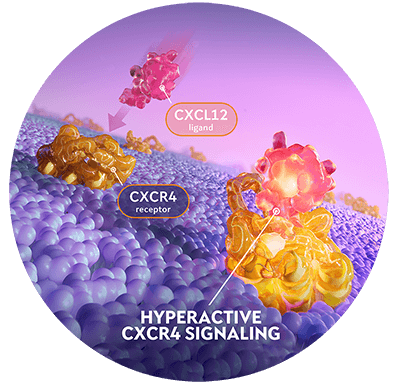
The underlying cause of WHIM syndrome is CXCR4 pathway dysregulation.4
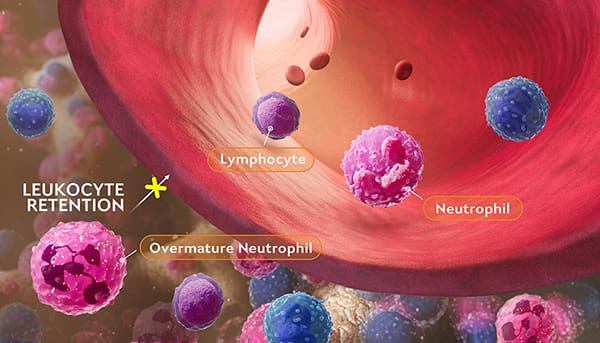
In WHIM syndrome, CXCR4 pathogenic variants cause hyperactive CXCR4 signaling. This leads to retention of leukocytes in the bone marrow.
Bone Marrow
Blood Vessel
XOLREMDI is an oral CXCR4 antagonist, and the first and only FDA-approved therapy for use in WHIM syndrome that targets CXCR4 pathway dysregulation to help improve immune function.4
Learn about XOLREMDICXCL12=CXC chemokine ligand 12. CXCR4=CXC chemokine receptor 4.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
XOLREMDI is contraindicated with drugs highly dependent on CYP2D6 for clearance.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity: Based on its mechanism of action, XOLREMDI is expected to cause fetal harm. Verify pregnancy status of female patients of reproductive potential prior to starting XOLREMDI. Advise females of reproductive potential to use effective contraception during treatment with XOLREMDI and for three weeks after the final dose.
QTc Interval Prolongation: XOLREMDI causes concentration-dependent QTc prolongation. Correct any modifiable risk factors for QTc prolongation, assess QTc at baseline, and monitor QTc during treatment as clinically indicated in patients with risk factors for QTc prolongation or receiving concomitant medications that increase XOLREMDI exposure and/or drugs with a known potential to prolong the QTc interval. Dose reduction or discontinuation of XOLREMDI may be required.
ADVERSE REACTIONS
The most common adverse reactions (in >10% patients and more frequently reported than placebo) were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
DRUG-DRUG INTERACTIONS
Avoid co-administration of XOLREMDI and strong CYP3A4 inducers. Reduce XOLREMDI daily dosage to 200 mg when administered with strong CYP3A4 inhibitors. Monitor more frequently for adverse reactions associated with an increase in exposure of XOLREMDI when used concomitantly with moderate CYP3A4 inhibitors or P-gp inhibitors and reduce XOLREMDI daily dosage if necessary.
USE IN SPECIFIC POPULATIONS
Advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose.
The safety and effectiveness of XOLREMDI have not been established in pediatric patients younger than 12 years of age.
XOLREMDI is not recommended in patients with severe renal impairment, end-stage renal disease, or moderate to severe hepatic impairment.
To report suspected adverse reactions, contact X4 Pharmaceuticals at 1-866-MED-X4MI (1-866-633-9464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
XOLREMDI® (mavorixafor) is a CXC chemokine receptor 4 (CXCR4) antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
Please see the full Prescribing Information for XOLREMDI.
References:
1. Dale DC, Firkin F, Bolyard AA, et al. Results of a phase 2 trial of an oral CXCR4 antagonist, mavorixafor, for treatment of WHIM syndrome. Blood. 2020;136(26):2994-3003. doi:10.1182/blood.2020007197
2. Geier CB, Ellison M, Cruz R, et al. Disease progression of WHIM syndrome in an international cohort of 66 pediatric and adult patients. J Clin Immunol. 2022;42(8):1748-1765. doi:10.1007/s10875-022-01312-7
3. Speckmann C, Doerken S, Aiuti A, et al. A prospective study on the natural history of patients with profound combined immunodeficiency: an interim analysis. J Allergy Clin Immunol. 2017;139(4):1302-1310.e4. doi:10.1016/j.jaci.2016.07.040
4. XOLREMDI. Prescribing information. X4 Pharmaceuticals Inc; 2024.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
XOLREMDI is contraindicated with drugs highly dependent on CYP2D6 for clearance.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity: Based on its mechanism of action, XOLREMDI is expected to cause fetal harm. Verify pregnancy status of female patients of reproductive potential prior to starting XOLREMDI. Advise females of reproductive potential to use effective contraception during treatment with XOLREMDI and for three weeks after the final dose.
QTc Interval Prolongation: XOLREMDI causes concentration-dependent QTc prolongation. Correct any modifiable risk factors for QTc prolongation, assess QTc at baseline, and monitor QTc during treatment as clinically indicated in patients with risk factors for QTc prolongation or receiving concomitant medications that increase XOLREMDI exposure and/or drugs with a known potential to prolong the QTc interval. Dose reduction or discontinuation of XOLREMDI may be required.
ADVERSE REACTIONS
The most common adverse reactions (in >10% patients and more frequently reported than placebo) were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
DRUG-DRUG INTERACTIONS
Avoid co-administration of XOLREMDI and strong CYP3A4 inducers. Reduce XOLREMDI daily dosage to 200 mg when administered with strong CYP3A4 inhibitors. Monitor more frequently for adverse reactions associated with an increase in exposure of XOLREMDI when used concomitantly with moderate CYP3A4 inhibitors or P-gp inhibitors and reduce XOLREMDI daily dosage if necessary.
USE IN SPECIFIC POPULATIONS
Advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose.
The safety and effectiveness of XOLREMDI have not been established in pediatric patients younger than 12 years of age.
XOLREMDI is not recommended in patients with severe renal impairment, end-stage renal disease, or moderate to severe hepatic impairment.
To report suspected adverse reactions, contact X4 Pharmaceuticals at 1-866-MED-X4MI (1-866-633-9464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
XOLREMDI® (mavorixafor) is a CXC chemokine receptor 4 (CXCR4) antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
Please see the full Prescribing Information for XOLREMDI.
References:
1. Dale DC, Firkin F, Bolyard AA, et al. Results of a phase 2 trial of an oral CXCR4 antagonist, mavorixafor, for treatment of WHIM syndrome. Blood. 2020;136(26):2994-3003. doi:10.1182/blood.2020007197
2. Geier CB, Ellison M, Cruz R, et al. Disease progression of WHIM syndrome in an international cohort of 66 pediatric and adult patients. J Clin Immunol. 2022;42(8):1748-1765. doi:10.1007/s10875-022-01312-7
3. Speckmann C, Doerken S, Aiuti A, et al. A prospective study on the natural history of patients with profound combined immunodeficiency: an interim analysis. J Allergy Clin Immunol. 2017;139(4):1302-1310.e4. doi:10.1016/j.jaci.2016.07.040
4. XOLREMDI. Prescribing information. X4 Pharmaceuticals Inc; 2024.
