IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
XOLREMDI is contraindicated with drugs highly dependent on CYP2D6 for clearance.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity: Based on its mechanism of action, XOLREMDI is expected to cause fetal harm. Verify pregnancy status of female patients of reproductive potential prior to starting XOLREMDI. Advise females of reproductive potential to use effective contraception during treatment with XOLREMDI and for three weeks after the final dose.
QTc Interval Prolongation: XOLREMDI causes concentration-dependent QTc prolongation. Correct any modifiable risk factors for QTc prolongation, assess QTc at baseline, and monitor QTc during treatment as clinically indicated in patients with risk factors for QTc prolongation or receiving concomitant medications that increase XOLREMDI exposure and/or drugs with a known potential to prolong the QTc interval. Dose reduction or discontinuation of XOLREMDI may be required.
ADVERSE REACTIONS
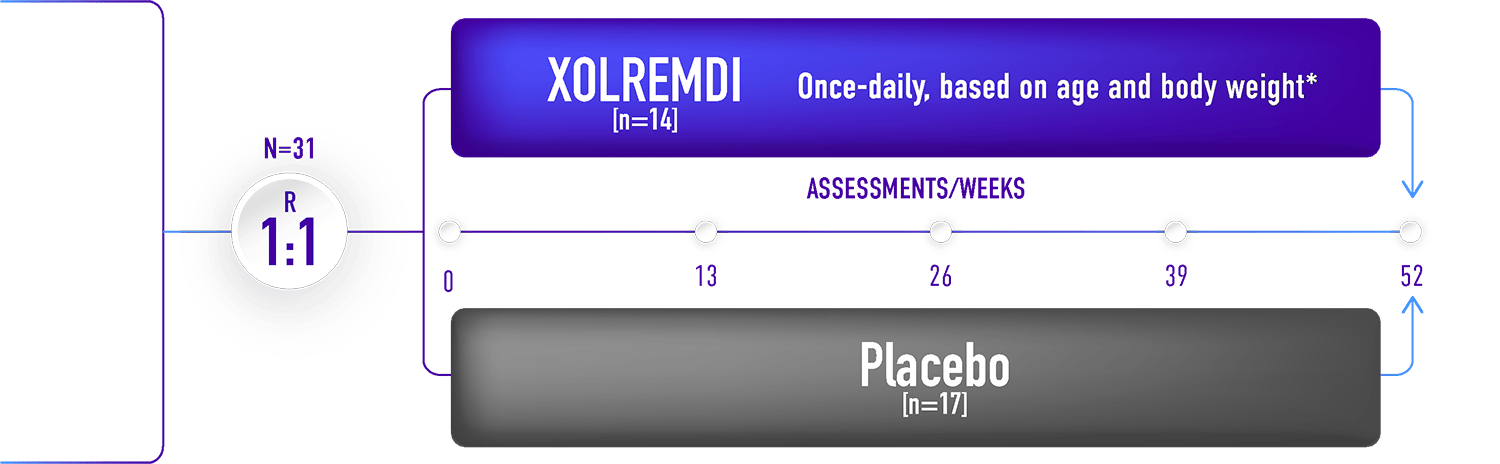
The most common adverse reactions (in >10% patients and more frequently reported than placebo) were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
DRUG-DRUG INTERACTIONS
Avoid co-administration of XOLREMDI and strong CYP3A4 inducers. Reduce XOLREMDI daily dosage to 200 mg when administered with strong CYP3A4 inhibitors. Monitor more frequently for adverse reactions associated with an increase in exposure of XOLREMDI when used concomitantly with moderate CYP3A4 inhibitors or P-gp inhibitors and reduce XOLREMDI daily dosage if necessary.
USE IN SPECIFIC POPULATIONS
Advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose.
The safety and effectiveness of XOLREMDI have not been established in pediatric patients younger than 12 years of age.
XOLREMDI is not recommended in patients with severe renal impairment, end-stage renal disease, or moderate to severe hepatic impairment.
To report suspected adverse reactions, contact X4 Pharmaceuticals at 1-866-MED-X4MI (1-866-633-9464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
XOLREMDI® (mavorixafor) is a CXC chemokine receptor 4 (CXCR4) antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
Please see the full Prescribing Information for XOLREMDI.
References:
1. XOLREMDI. Prescribing information. X4 Pharmaceuticals Inc; 2024.
2. Badolato R, Alsina L, Azar A, et al. Phase 3 randomized trial of mavorixafor, CXCR4 antagonist, in WHIM syndrome. Blood. Published online April 21, 2024. doi:10.1182/blood.2023022658
3. Boxer LA. How to approach neutropenia. Hematology Am Soc Hematol Educ Program. 2012;2012:174-182. doi:10.1182/asheducation-2012.1.174
4. McDermott DH, Murphy PM. WHIM syndrome: immunopathogenesis, treatment and cure strategies. Immunol Rev. 2019;287(1):91-102. doi:10.1111/imr.12719
5. Sicre de Fontbrune F, Moignet A, Beaupain B, et al. Severe chronic primary neutropenia in adults: report on a series of 108 patients. Blood. 2015;126(14):1643-1650. doi:10.1182/blood-2015-03-634493