XOLREMDI efficacy data1
XOLREMDI significantly increased time above threshold (TAT) for ANC and ALC compared with placebo, suggestive of improved immune function.1
XOLREMDI improved absolute neutrophil and absolute lymphocyte counts (ANC and ALC).1
- ANC below 500 cells/μL (severe neutropenia) and ALC below 1,000 cells/μL (lymphopenia) is associated with increased infection risk.2,3,4
- ANC and ALC peaked at 4 hours after dosing and returned toward baseline within 24 hours.1
Mean Time Over 24 Hours That ANC Was Above 500 cells/µL (TATANC)*,1
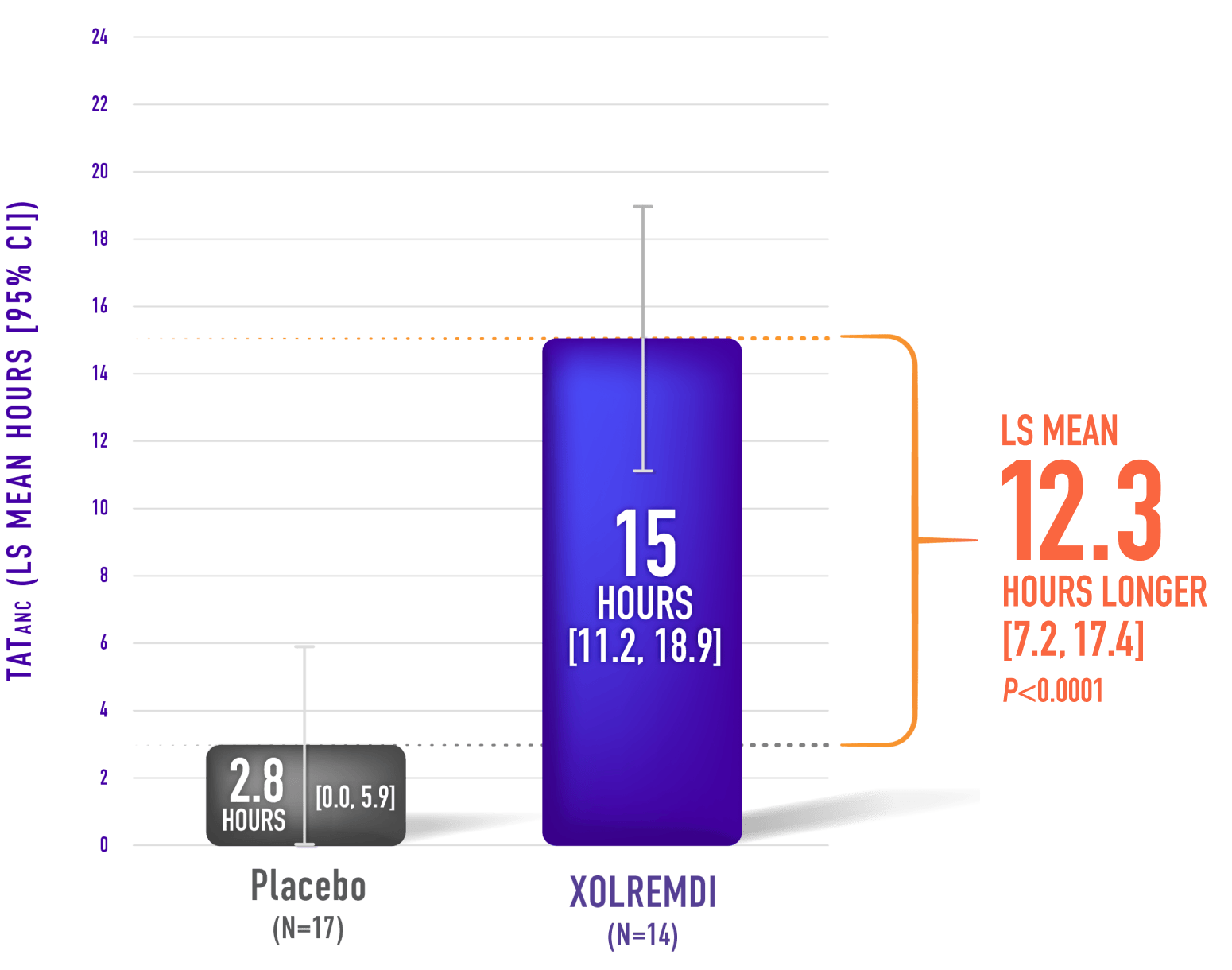
XOLREMDI significantly decreased the time that patients had severe neutropenia compared with placebo1,2
Mean Time Over 24 Hours That ALC Was Above 1,000 cells/µL (TATALC)*,1,5
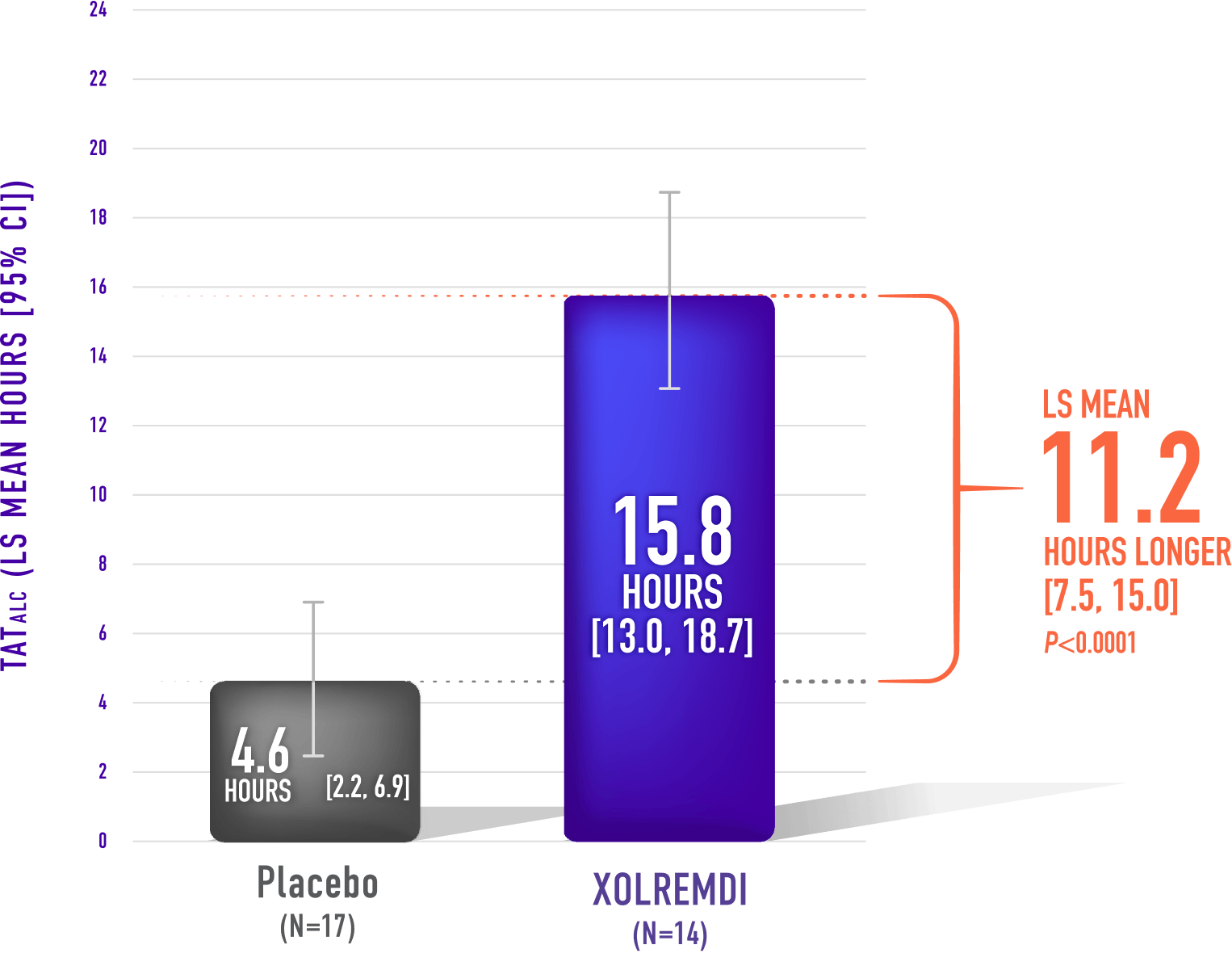
XOLREMDI significantly decreased the time that patients had lymphopenia compared with placebo1,6
Demonstrated clinical benefit with XOLREMDI1
Win-Ratio analysis of a composite endpoint showed superior clinical efficacy in patients treated with XOLREMDI compared with placebo.
Win-Ratio analysis for the composite endpoint based on a pair-wise comparison of patients based on hierarchical evaluation of total infection score† and total wart change score‡
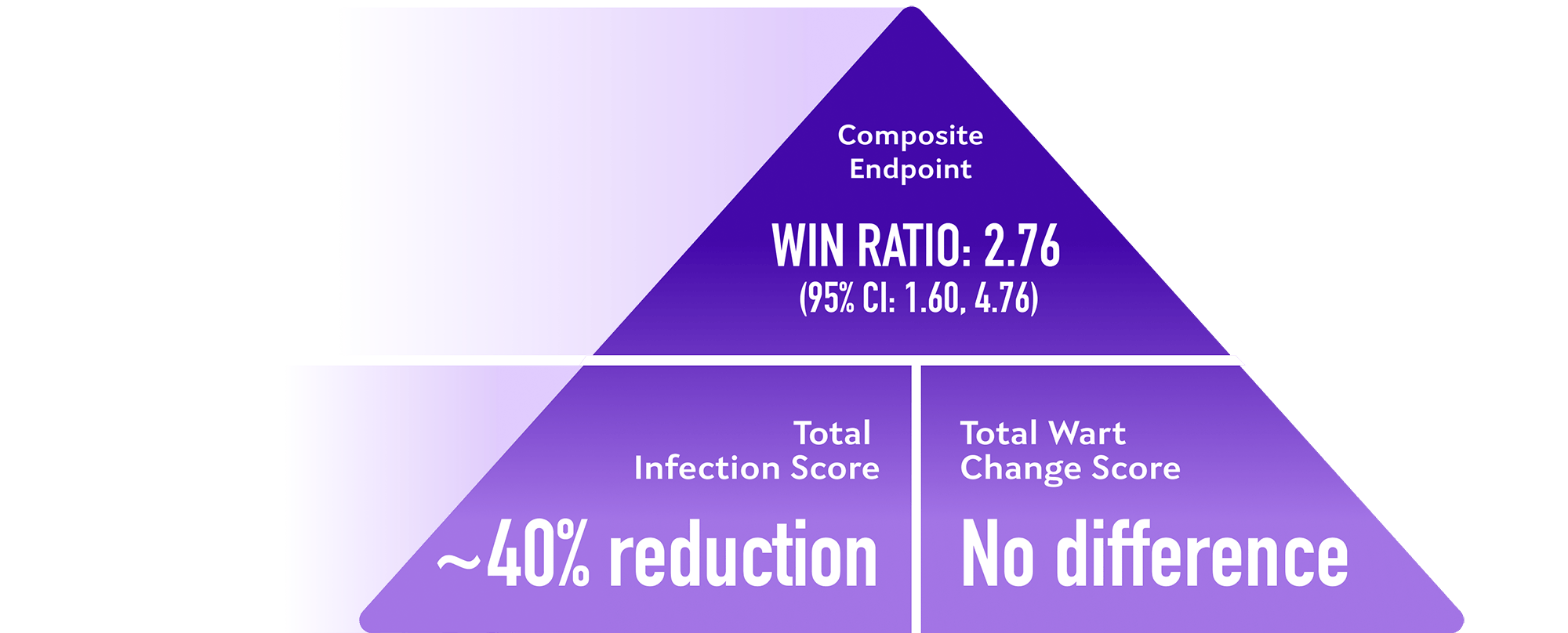
Pre-specified analyses of composite endpoint components, XOLREMDI compared with placebo
Patients treated with XOLREMDI experienced fewer infections compared with placebo over 1 year.1
Annualized Infection Rate
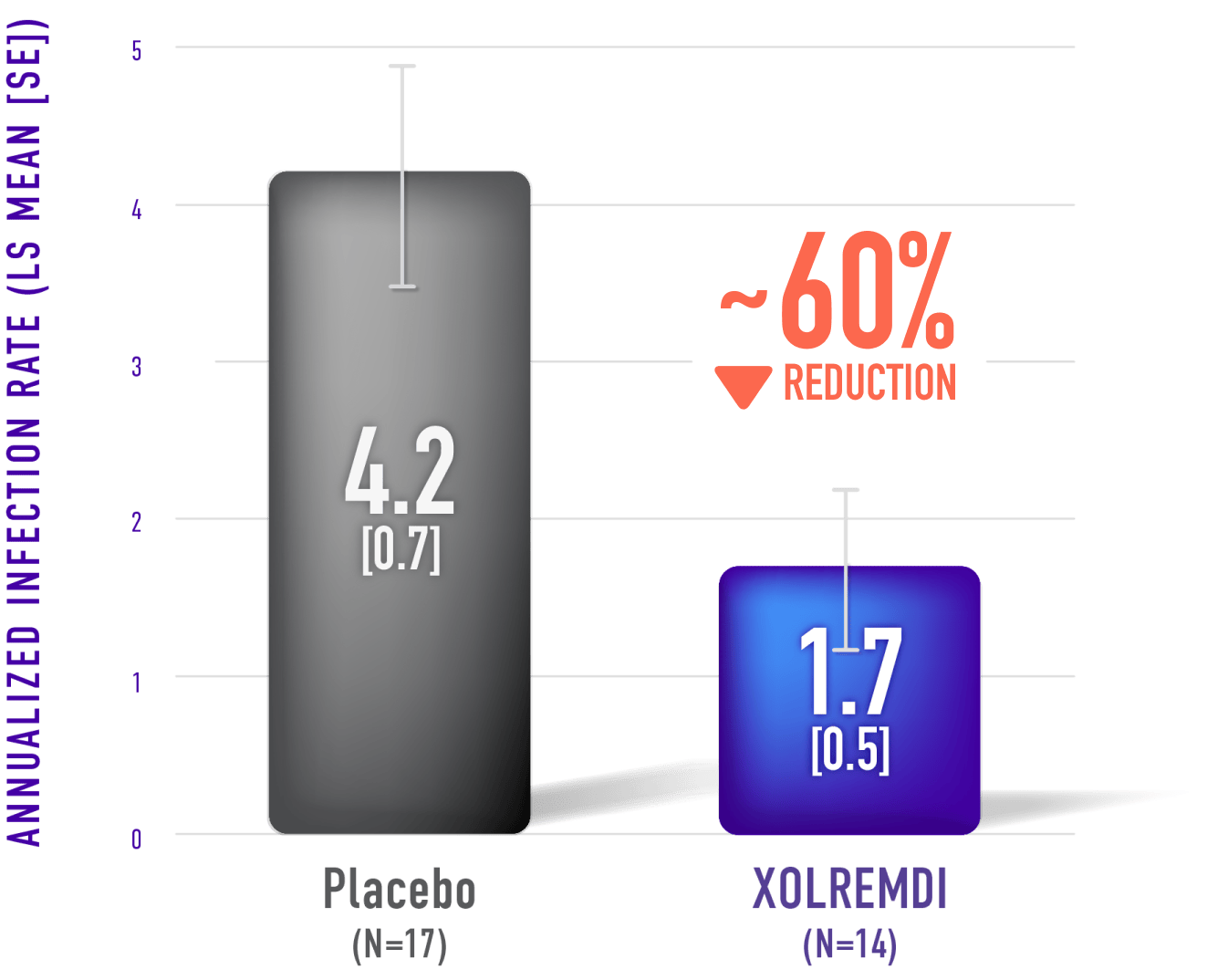
XOLREMDI reduced the annualized infection rate by approximately 60% compared with placebo1
ALC=absolute lymphocyte count. ANC=absolute neutrophil count. CI=confidence interval. LS=least squares. SE=standard error. TAT=time above threshold.
*Assessed 4 times throughout the 52-week study (once every 3 months).
†The total infection score was calculated by summing up the number of infection events weighted by severity and divided by the total exposure time (in years).
‡The total wart change score was calculated by summing up the regional wart change scores from 3 target regions (lesions).
How was XOLREMDI studied?
XOLREMDI was studied in a randomized, double-blind, placebo-controlled, Phase 3 trial of 31 patients with WHIM syndrome.1,5
Getting Started
Start prescribing and connect your patient to support resources with X4Connect.
Prescribe XOLREMDIIMPORTANT SAFETY INFORMATION
CONTRAINDICATION
XOLREMDI is contraindicated with drugs highly dependent on CYP2D6 for clearance.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity: Based on its mechanism of action, XOLREMDI is expected to cause fetal harm. Verify pregnancy status of female patients of reproductive potential prior to starting XOLREMDI. Advise females of reproductive potential to use effective contraception during treatment with XOLREMDI and for three weeks after the final dose.
QTc Interval Prolongation: XOLREMDI causes concentration-dependent QTc prolongation. Correct any modifiable risk factors for QTc prolongation, assess QTc at baseline, and monitor QTc during treatment as clinically indicated in patients with risk factors for QTc prolongation or receiving concomitant medications that increase XOLREMDI exposure and/or drugs with a known potential to prolong the QTc interval. Dose reduction or discontinuation of XOLREMDI may be required.
ADVERSE REACTIONS
The most common adverse reactions (in >10% patients and more frequently reported than placebo) were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
DRUG-DRUG INTERACTIONS
Avoid co-administration of XOLREMDI and strong CYP3A4 inducers. Reduce XOLREMDI daily dosage to 200 mg when administered with strong CYP3A4 inhibitors. Monitor more frequently for adverse reactions associated with an increase in exposure of XOLREMDI when used concomitantly with moderate CYP3A4 inhibitors or P-gp inhibitors and reduce XOLREMDI daily dosage if necessary.
USE IN SPECIFIC POPULATIONS
Advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose.
The safety and effectiveness of XOLREMDI have not been established in pediatric patients younger than 12 years of age.
XOLREMDI is not recommended in patients with severe renal impairment, end-stage renal disease, or moderate to severe hepatic impairment.
To report suspected adverse reactions, contact X4 Pharmaceuticals at 1-866-MED-X4MI (1-866-633-9464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
XOLREMDI® (mavorixafor) is a CXC chemokine receptor 4 (CXCR4) antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
Please see the full Prescribing Information for XOLREMDI.
References:
1. XOLREMDI. Prescribing information. X4 Pharmaceuticals Inc; 2024.
2. Boxer LA. How to approach neutropenia. Hematology. 2012;2012(1):174-182. doi:10.1182/asheducation.v2012.1.174.3798251
3. Sicre de Fontbrune F, Moignet A, Beaupain B, et al. Severe chronic primary neutropenia in adults: report on a series of 108 patients. Blood. 2015;126(14):1643-1650. doi:10.1182/blood-2015-03-634493
4. Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. 2018;15(11):e1002685. Published 2018 Nov 1. doi:10.1371/journal.pmed.1002685
5. Badolato R, Alsina L, Azar A, et al. Phase 3 randomized trial of mavorixafor, CXCR4 antagonist, in WHIM syndrome. Blood. Published online April 21, 2024. doi:10.1182/blood.2023022658
6. Speckmann C, Doerken S, Aiuti A, et al. A prospective study on the natural history of patients with profound combined immunodeficiency: an interim analysis. J Allergy Clin Immunol. 2017;139(4):1302-1310.e4. doi:10.1016/j.jaci.2016.07.040
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
XOLREMDI is contraindicated with drugs highly dependent on CYP2D6 for clearance.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity: Based on its mechanism of action, XOLREMDI is expected to cause fetal harm. Verify pregnancy status of female patients of reproductive potential prior to starting XOLREMDI. Advise females of reproductive potential to use effective contraception during treatment with XOLREMDI and for three weeks after the final dose.
QTc Interval Prolongation: XOLREMDI causes concentration-dependent QTc prolongation. Correct any modifiable risk factors for QTc prolongation, assess QTc at baseline, and monitor QTc during treatment as clinically indicated in patients with risk factors for QTc prolongation or receiving concomitant medications that increase XOLREMDI exposure and/or drugs with a known potential to prolong the QTc interval. Dose reduction or discontinuation of XOLREMDI may be required.
ADVERSE REACTIONS
The most common adverse reactions (in >10% patients and more frequently reported than placebo) were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
DRUG-DRUG INTERACTIONS
Avoid co-administration of XOLREMDI and strong CYP3A4 inducers. Reduce XOLREMDI daily dosage to 200 mg when administered with strong CYP3A4 inhibitors. Monitor more frequently for adverse reactions associated with an increase in exposure of XOLREMDI when used concomitantly with moderate CYP3A4 inhibitors or P-gp inhibitors and reduce XOLREMDI daily dosage if necessary.
USE IN SPECIFIC POPULATIONS
Advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose.
The safety and effectiveness of XOLREMDI have not been established in pediatric patients younger than 12 years of age.
XOLREMDI is not recommended in patients with severe renal impairment, end-stage renal disease, or moderate to severe hepatic impairment.
To report suspected adverse reactions, contact X4 Pharmaceuticals at 1-866-MED-X4MI (1-866-633-9464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
XOLREMDI® (mavorixafor) is a CXC chemokine receptor 4 (CXCR4) antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
Please see the full Prescribing Information for XOLREMDI.
References:
1. XOLREMDI. Prescribing information. X4 Pharmaceuticals Inc; 2024.
2. Boxer LA. How to approach neutropenia. Hematology. 2012;2012(1):174-182. doi:10.1182/asheducation.v2012.1.174.3798251
3. Sicre de Fontbrune F, Moignet A, Beaupain B, et al. Severe chronic primary neutropenia in adults: report on a series of 108 patients. Blood. 2015;126(14):1643-1650. doi:10.1182/blood-2015-03-634493
4. Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. 2018;15(11):e1002685. Published 2018 Nov 1. doi:10.1371/journal.pmed.1002685
5. Badolato R, Alsina L, Azar A, et al. Phase 3 randomized trial of mavorixafor, CXCR4 antagonist, in WHIM syndrome. Blood. Published online April 21, 2024. doi:10.1182/blood.2023022658
6. Speckmann C, Doerken S, Aiuti A, et al. A prospective study on the natural history of patients with profound combined immunodeficiency: an interim analysis. J Allergy Clin Immunol. 2017;139(4):1302-1310.e4. doi:10.1016/j.jaci.2016.07.040
